Ocean Acidification
Summary
Ocean acidification (OA) results from the additional inputs of carbon dioxide (CO2) from human activities. Photosynthesis by plants and bacteria uses CO2, creating oceanic sinks of carbon. The vast open oceans are teaming with photosynthetic plankton. However, there are practical limits to plant growth that arise from temporal scales and water chemistry. The carbon taken up by plants, and further incorporated into animals, only temporarily stores carbon. As flora and fauna die, bacteria release CO2 in the decomposition process. Therefore, this sequestration method does not match the time-frame or cumulative energy of processes involved in creating fossil fuels or appreciably lowering atmospheric carbon concentrations. Carbon dioxide (CO2) is considered a pollutant for the fact that there is no comparable anthropogenic uptake mechanism to compensate for anthropogenic emissions.
Chemistry
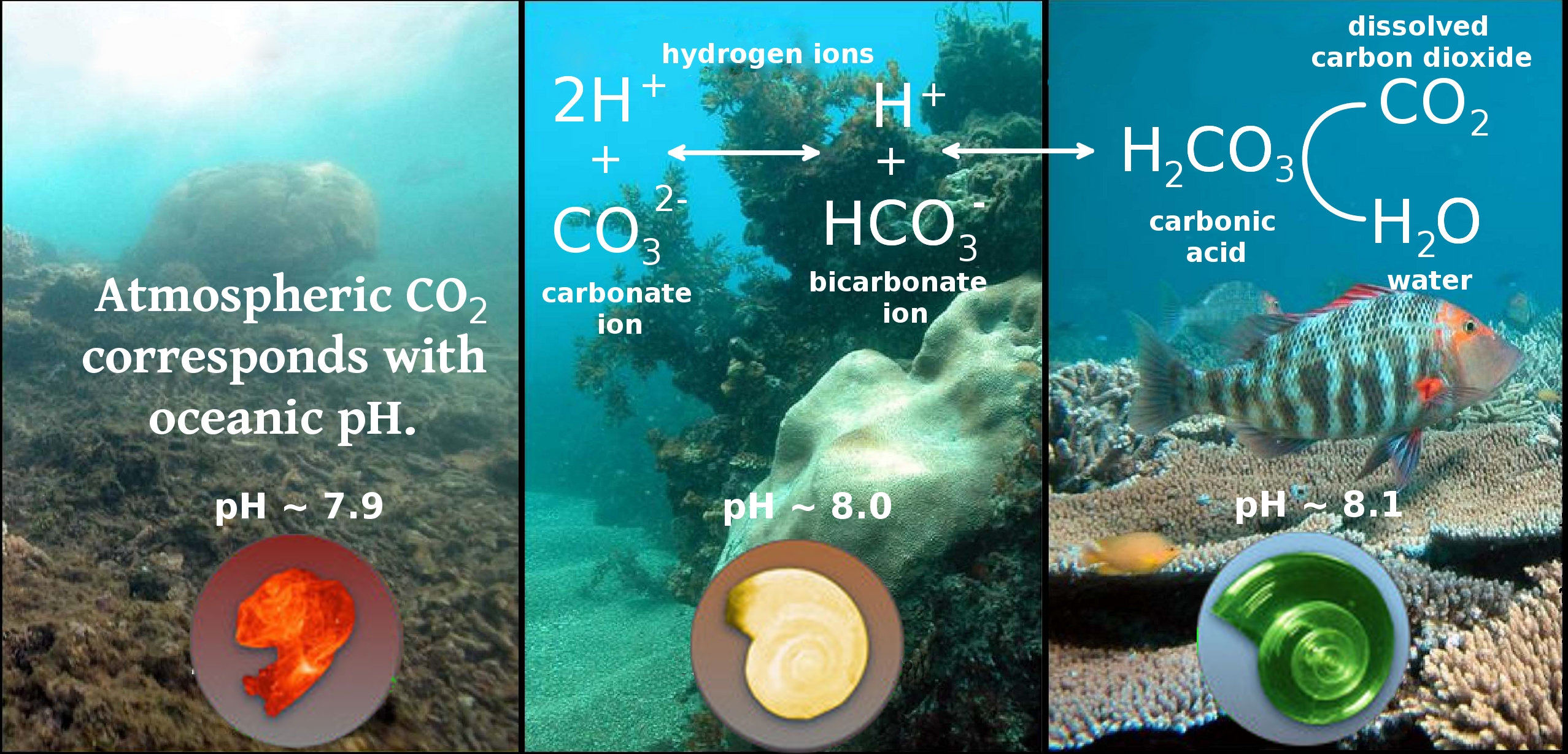
Ocean Acidification (OA) refers to exactly what it sounds like, oceans acidifying. The same acid-base relationship seen between lemon juice and bleach, less than pH 7 is considered acidic and greater than pH 7 is considered basic, or alkaline. This relationship is signified by the pH scale (Figure 2) and is a measurement of hydrogen ion concentrations ([H+]), described by the following equation: pH = -log[H+] Two things to note when evaluating this equation, the fact that it's negative and it's log scale. The log scale means that [H+] vary exponentially and that the log is negative indicates the more H+ ions you have the lower the value. A solution with low [H+] has a high pH and is considered basic, high [H+] are reflected by a low pH and is considered acidic. The pH scale range is between 0 and 14, and distinguishes the concentrations of H+ and corresponding hydroxide (OH-) ions.
Figure 2, shows on top, a depiction of the relative concentrations of [H+] and [OH-] ions for pH range 6-8, around the ph 7 midpoint; on the bottom, relative [H+] to pH 7 of 'pure' water and a list of pH values of corresponding common substances, biological tolerances, and source waters. River water is shown to have pH values between 7 and 7.5, and rain water between 5 and 5.6. Ocean waters have a pH around 8.2 that varies by about 0.3 units depending on seasonal and diurnal patterns, regional variations, and local conditions. Ocean water is shown as having a pH of between 8 and 8.4. While oceanic water is generally above pH 7 and is considered basic, OA is the actual change in ocean chemistry to reflect that the ocean is becoming more acidic with anthropogenic emissions.
The increasing carbon dioxide concentrations in the atmosphere are subject to Henry's Law which states that 'at a constant temperature the solubility of a gas in a liquid is proportional to the partial pressure of the gas in contact with the liquid', meaning the increasing carbon dioxide in the atmosphere increases carbon dioxide in the oceans. As carbon dioxide dissolves in water it acts as a weak acid which forms carbonic acid. In slightly alkaline oceanic waters (pH ~ 8.2), only a small portion remains as carbonic acid. Carbon dioxide increases the [H+] concentrations in the ocean, reducing the pH. Carbonic acid is in equilibrium with bicarbonate and carbonate ions (see Equation 2). Concentrations of each ion species are controlled by environmental conditions like temperature, salinity, and partial pressures of the gases involved, known as equilibrium constants when used in modeling. Dissolution of carbon dioxide in the ocean increases concentrations of hydrogen ion and decreases concentrations of carbonate ion. This equilibrium between carbonaceous ion species is represented by the graph in Figure 3 and can be seen in the following equation: carbon dioxide + water ? carbonic acid ? bicarbonate ion ? carbonate ion (2)
Figure 3 represents the carbonate species in water according to pH range 7 - 9 and the equilibrium is calculated for standard temperature (25 degrees Celsius), salinity (35 parts per thousand), and pressure (1 atmosphere). This pH range covers typical oceanic water. The variation in carbonate species is why this equilibrium is considered a buffer, it allows additional input of ions and the pH varies accordingly on a exponential scale. The most relevant aspect to Figure 3 is that the highest concentrations of carbon dioxide are associated with the lowest pH. Recalling that dissolved carbon dioxide concentrations in the oceans respond to atmospheric concentrations, with corresponding lower pH levels that have ramifications on multiple aspects of oceanic life, addressing anthropogenic carbon emissions is vital to sustainability.
Projected Changes in Ocean Chemistry from Historical Record
Table 1 describes the changes the ocean experiences in responses to increasing atmospheric carbon dioxide. The changes described are carbonate ion concentrations, carbonate saturation values, total dissolved inorganic carbon, and the average pH of oceanic surface waters. It can be seen clearly, according to well know chemistry of these common substances, as atmospheric carbon dioxide concentrations rise the associated total dissolved inorganic carbon also rises and pH drops.

It has been estimated, independent from the results of the OCMIP3 model presented in Table 1, that there has been a 0.1 drop in the world's oceanic pH since the beginning of the industrial revolution. The Pacific Marine Environmental Laboratory in Hawaii has a substantial record of atmospheric carbon dioxide since the mid 1950's. This data is presented with seawater pH and oceanic carbon dioxide pressures in Figure 4.
It can be seen in Figure 4 that atmospheric and oceanic carbon dioxide concentrations rise in a similar fashion as seawater pH drops. This graph aligns with Henry's Law and the known chemistry of seawater. Future changes in these parameters, described in Table 1, indicate the distinct possibility of major disruption to biological processes if anthropogenic carbon emissions can not be mitigated. The degree to which inner coastal processes and specific marine species are related to the calcium carbonate equilibrium are not as well understood but are being actively researched.